Why the FDA barred so many KN95 masks
Testing revealed quality control issues or low effectiveness
by KEVIN KUO DDS, MMSc | May 10, 2020
The Food and Drug Administration (FDA) recently removed most KN95 manufacturers from its Emergency Use Authorization (EUA) list for non-NIOSH-approved respirators imported from China. Listed respirators were allowed to be used by healthcare personnel in emergency or crisis times, like the COVID-19 pandemic. The Agency cited poor quality as the reason for the change.
The National Personal Protective Technology Laboratory (NPPTL) is the division within the National Institute for Occupational Safety and Health (NIOSH) responsible for conducting research ensuring the safety of personal protective equipment. In the past several weeks, the NPPTL has been assessing respirators submitted by United States workplaces, such as healthcare systems or other government agencies. These tests were meant to be point-of-use assessments and not substitutes for full NIOSH-approval. Tests are modified versions of the NIOSH Standard Test Procedure. Ten respirators per manufacturers were randomly assessed, and only the particulate filter efficiency level was tested in the following parameters:
a. The flow rate will be set to 85.0 ± 4.0 Liters/Minute.
b. Aerosol concentration will not exceed 200 mg/m3 .
c. The particle size distribution will be 0.075 ± 0.020 micrometer with a geometric standard deviation not exceeding 1.86. 7 | Page
d. Each respirator will be tested for 10 minutes.
e. Maximum penetration will be recorded for each individual respirator
As of May 10th, the NPPTL tested a total of 111 different respirators, including the 7 from the April 3rd EUA list. Forty-three (43) manufacturers had significant filtration failures (less than 80%) in some or all of their respirators. They demonstrated inefficient filtration efficiency and/or quality control. Forty-one (41) manufacturers passed and filtered more than 95% in all 10 units. Those manufacturers who passed all of their respirators do not receive automatic approval to the EUA list due to other criteria necessary.
The results of the seven Chinese manufacturers who were previously on the EUA list that failed are listed below:
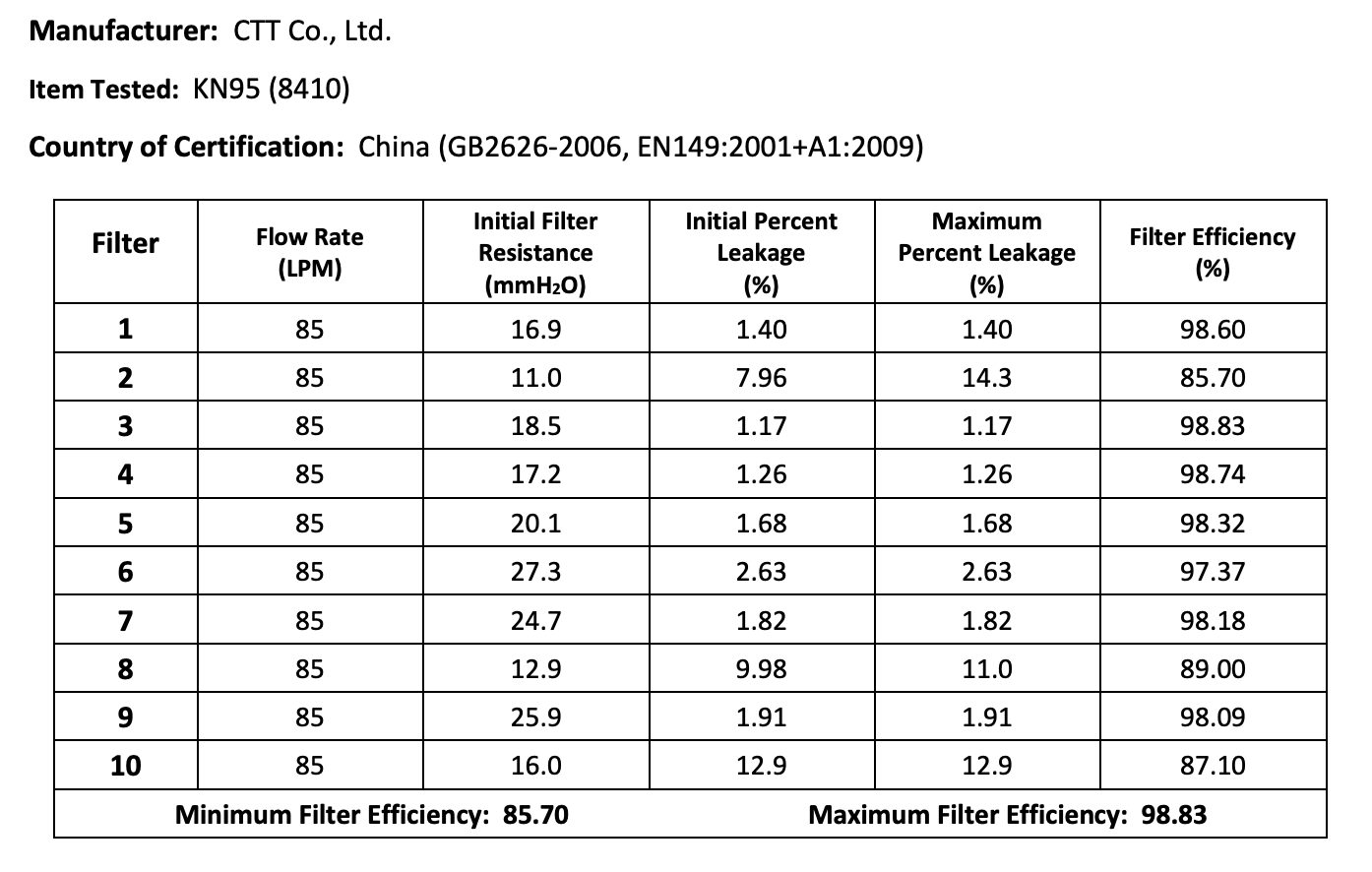
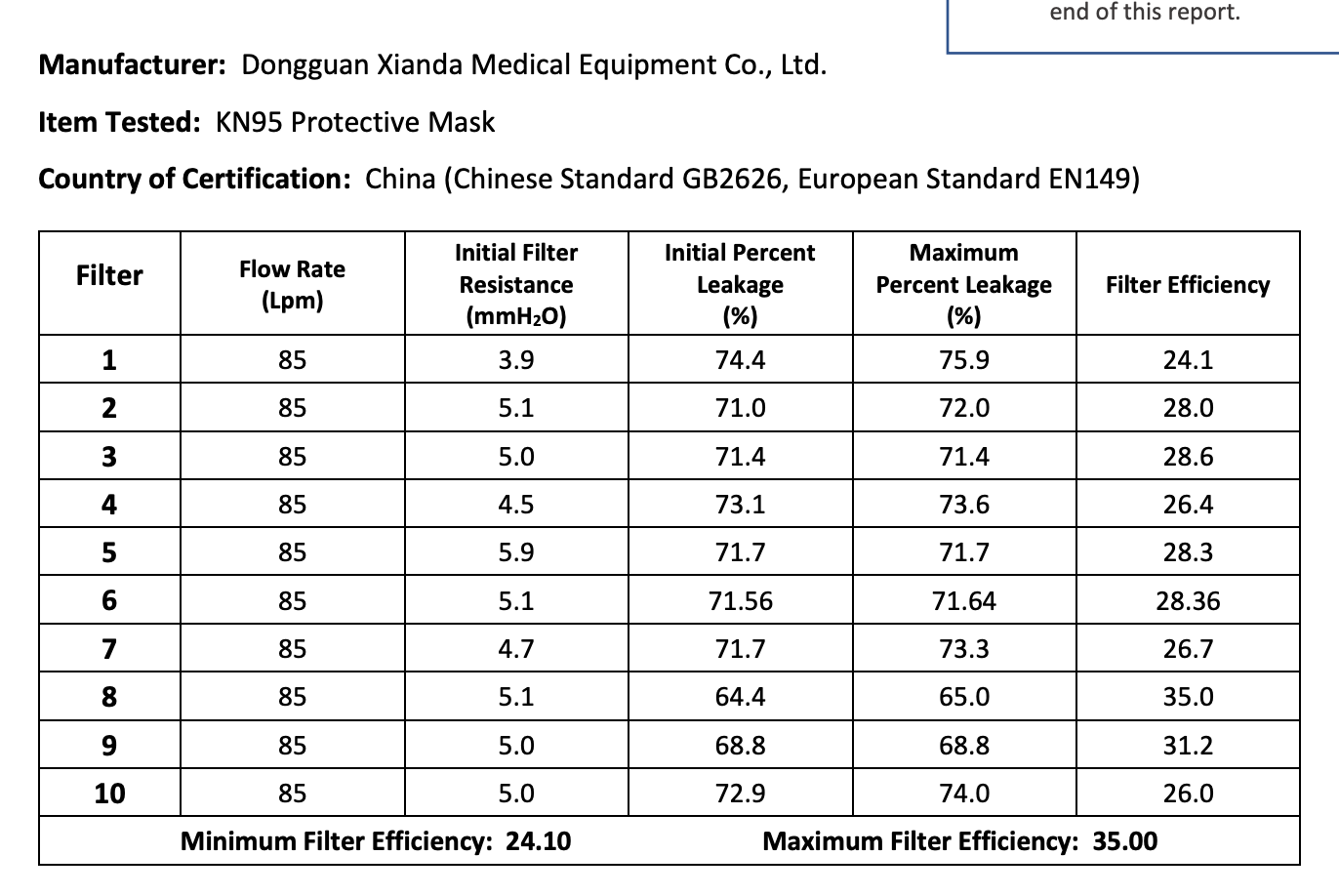
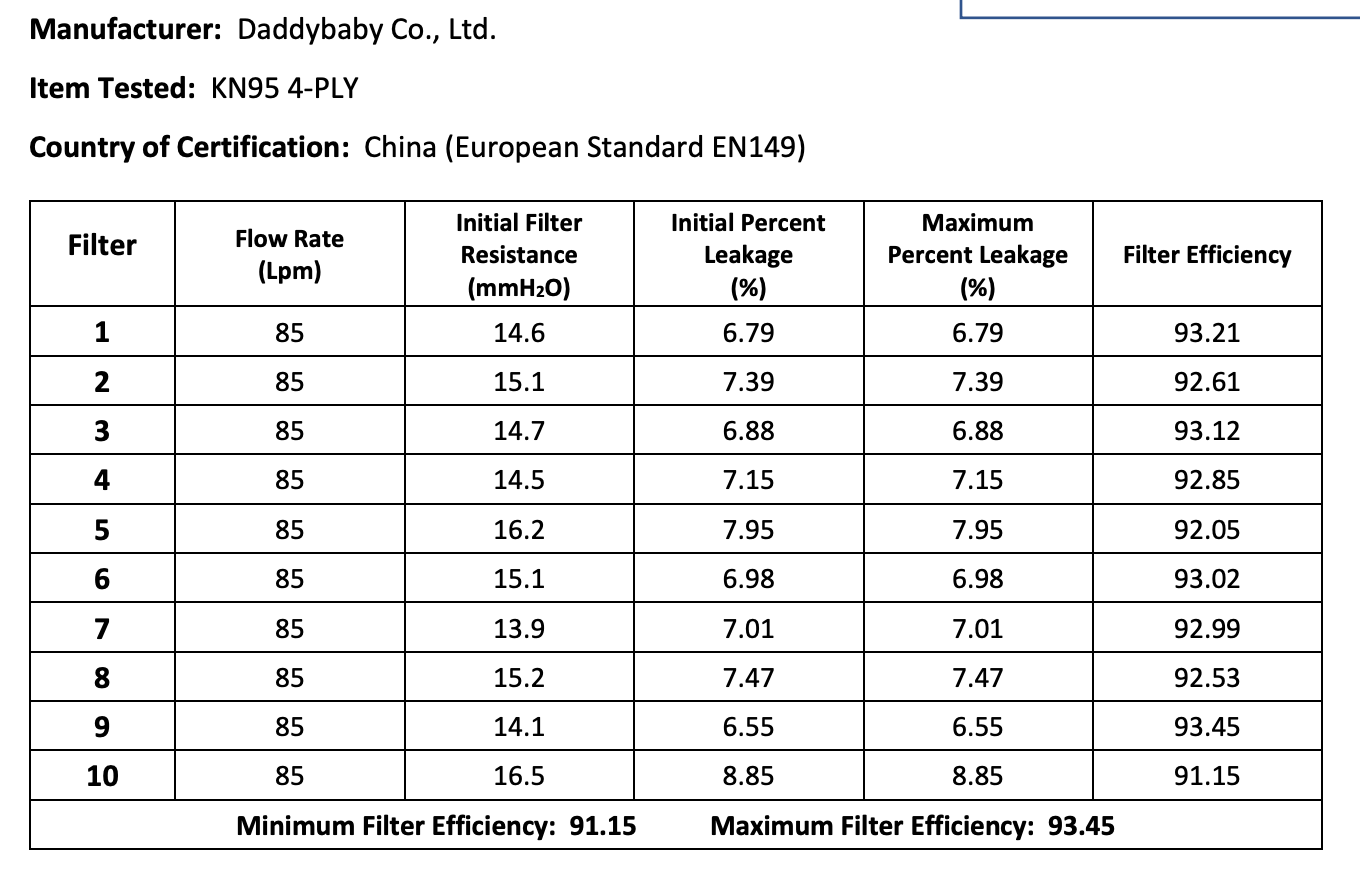
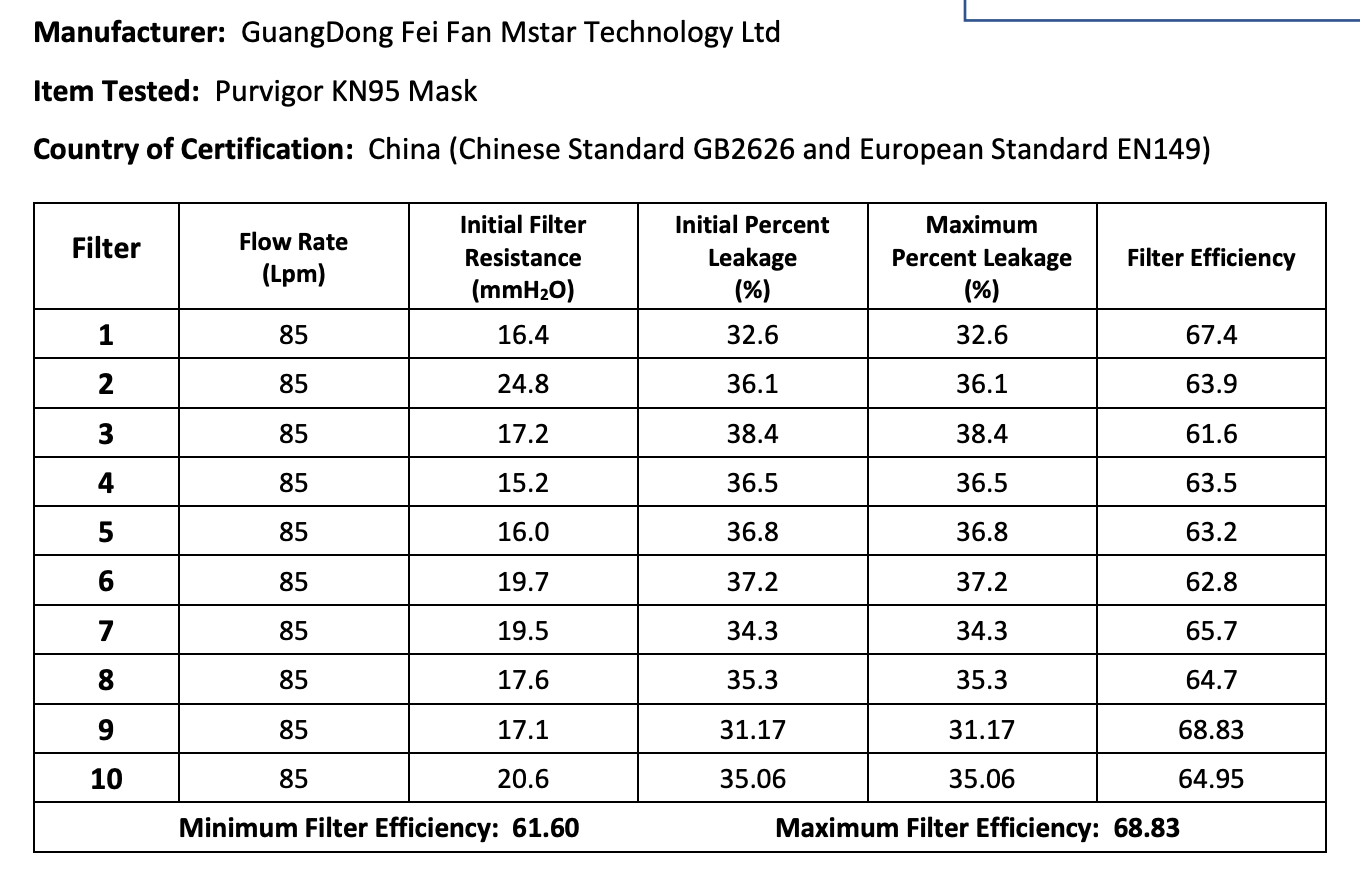
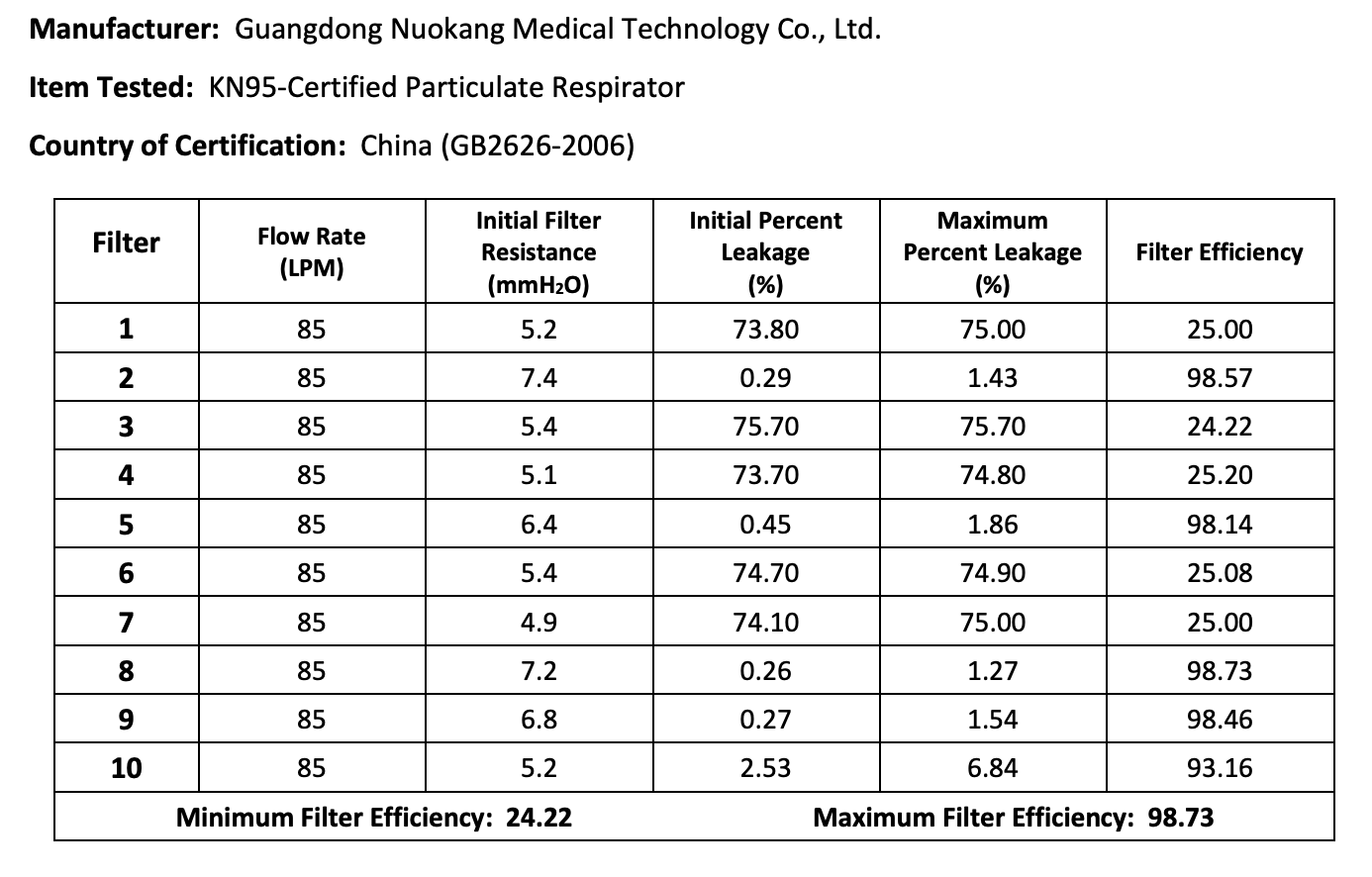
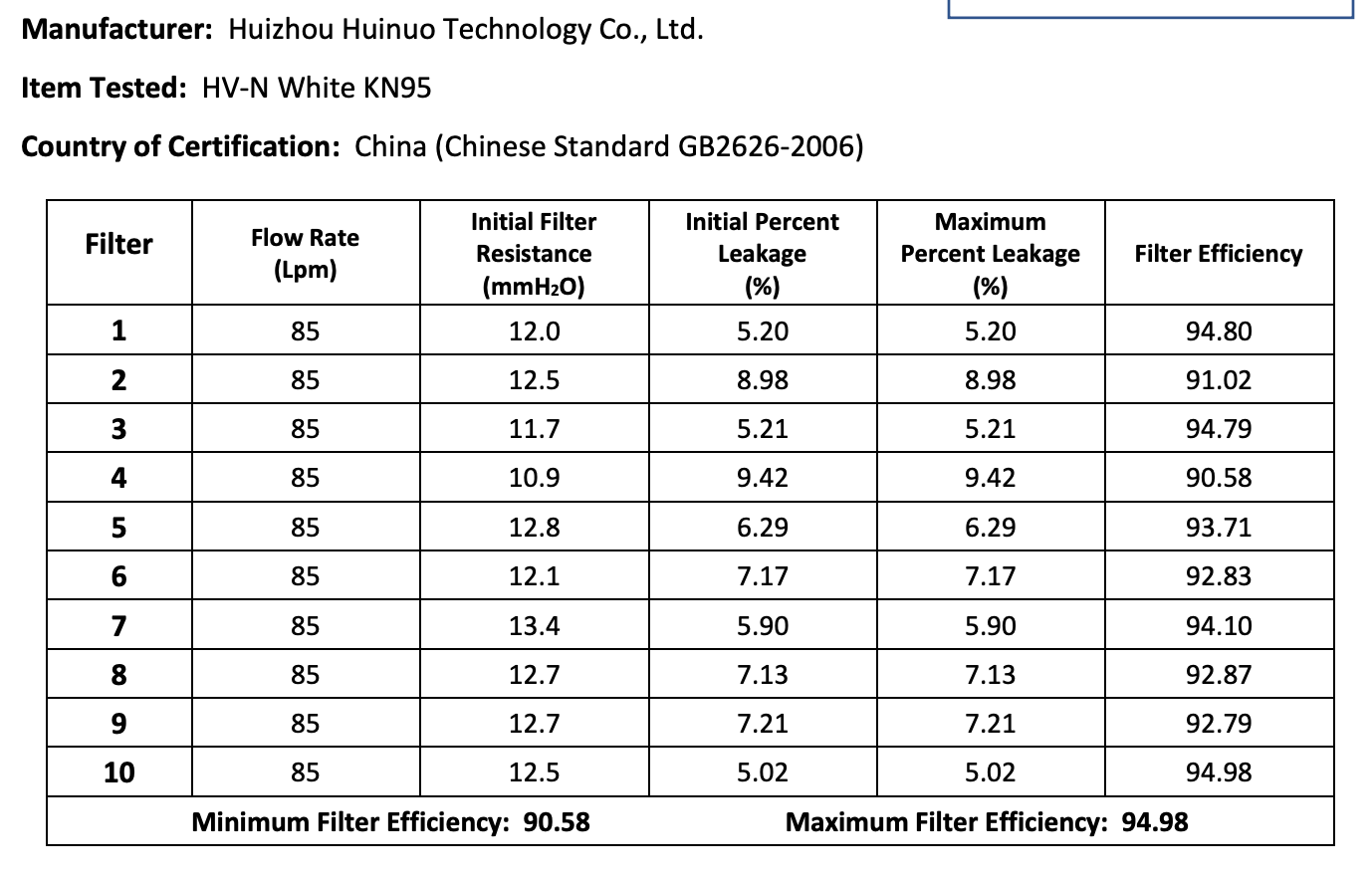
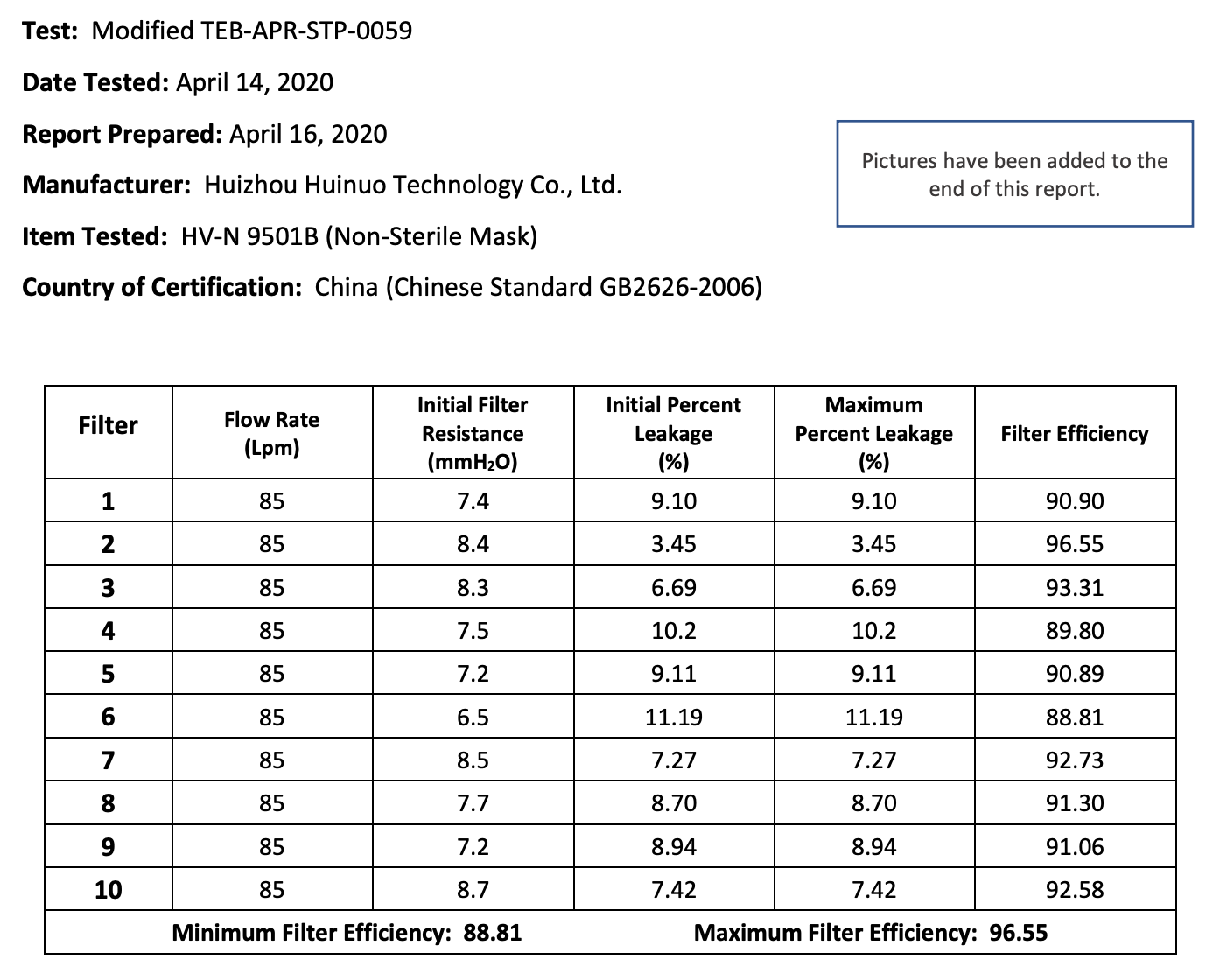
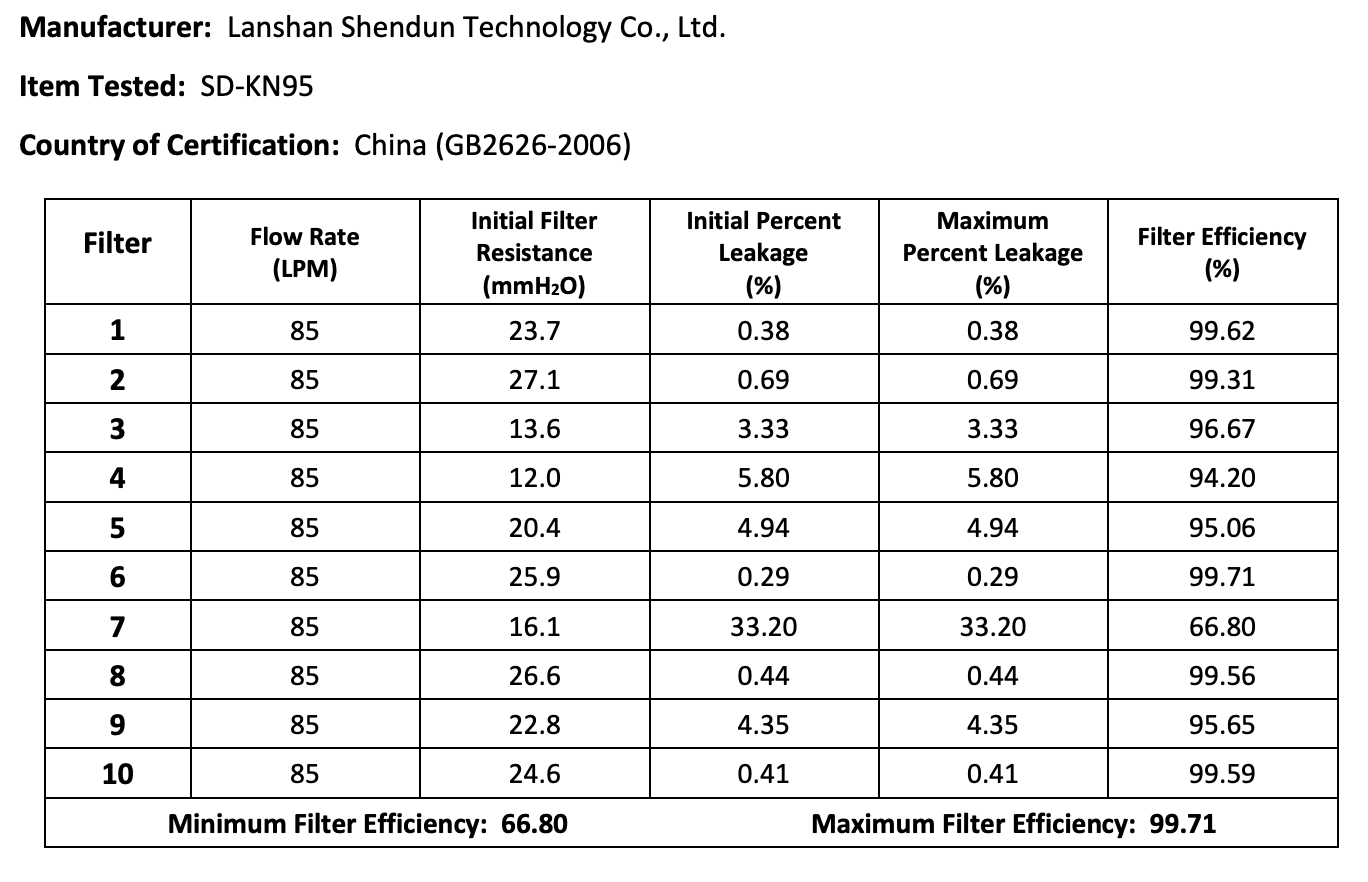
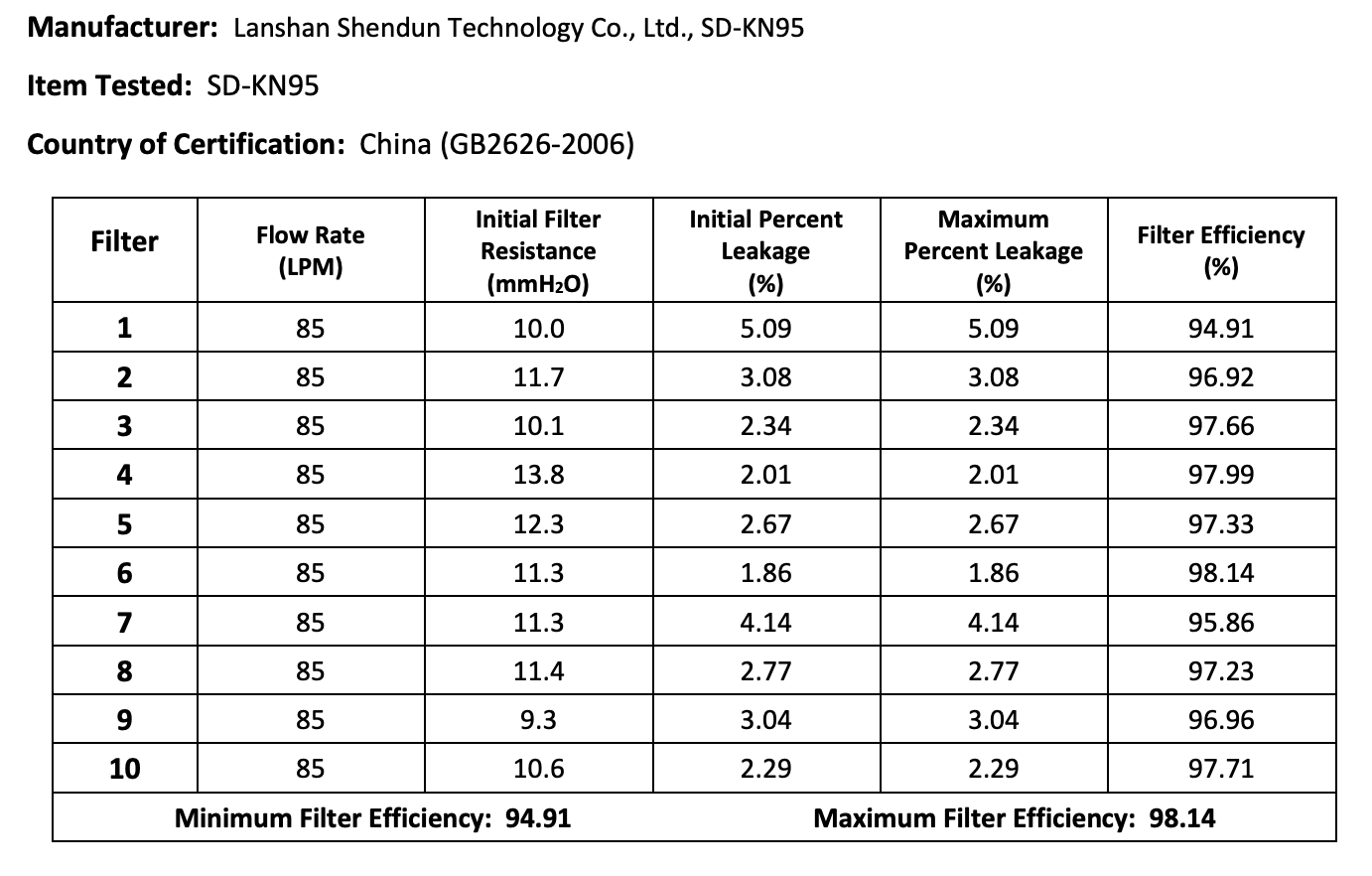
Results summary by manufacturer compiled here:
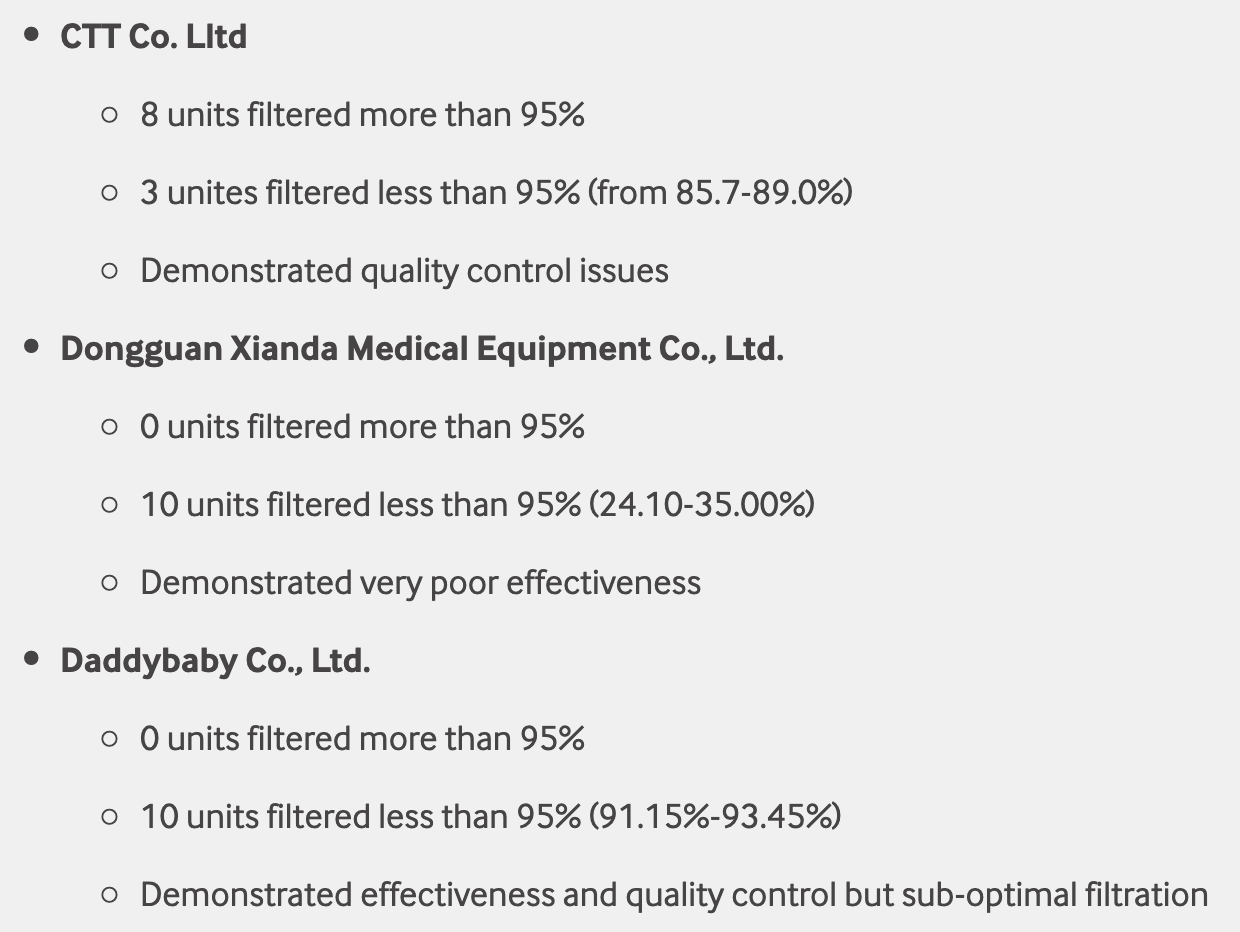

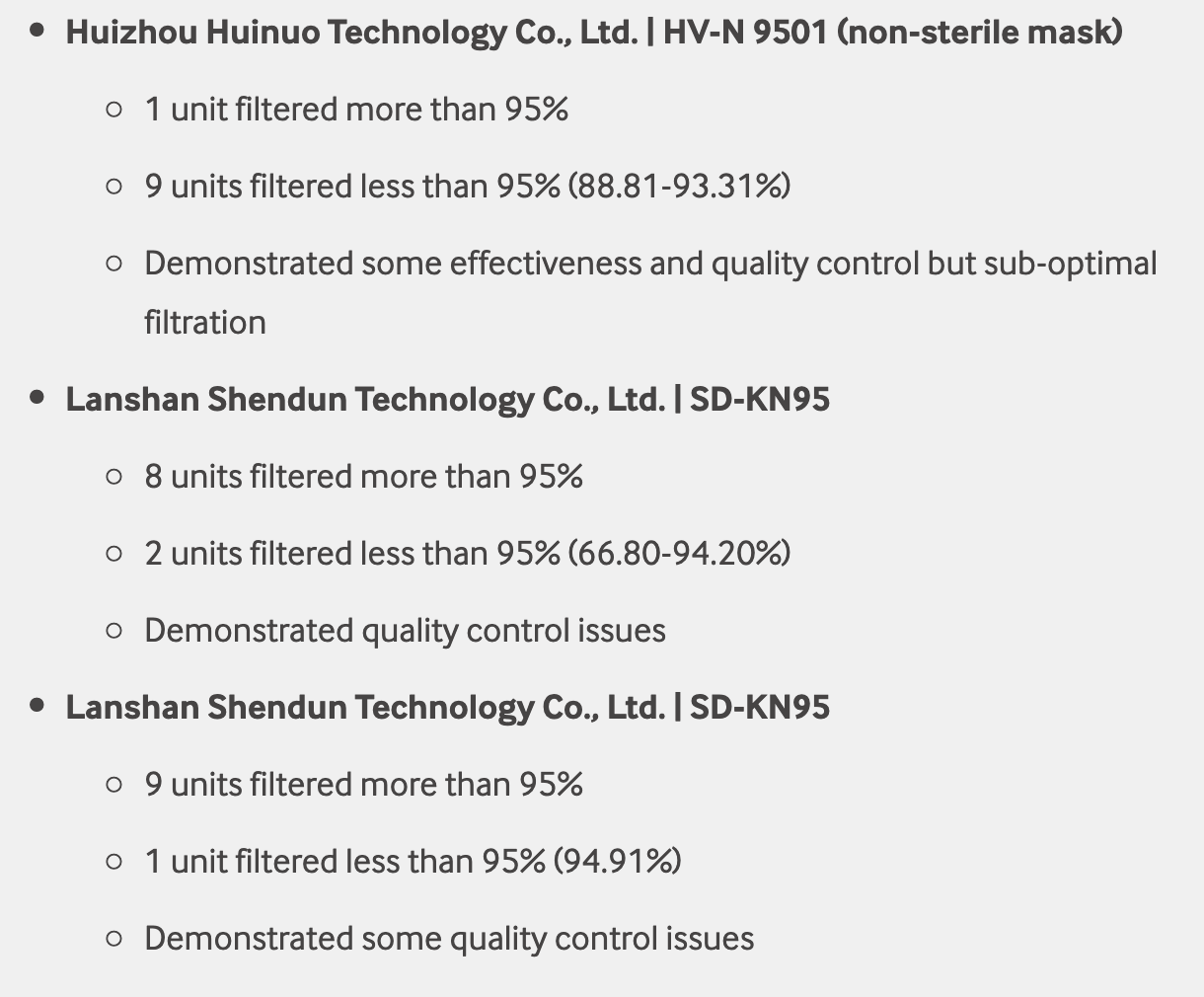
Test failures varied significantly depending on which of the seven manufacturers. Some failed due to insufficient filtration considerably below 95% for all units tested. Some failed due to inconsistent filtration above 95%. Other failed due to slightly below 95% filtration. This variability was the case for the rest of the other 104 manufacturers tested.
The FDA most likely decided to remove all non-NTTPL-tested manufacturers from the EUA list due to the wide range of test results from the seven manufacturers above and the many others. NIOSH-testing is now required to be re-approved or accepted.





