FDA blocks most KN95 masks from approved list
Bad news for prepared dentists seeking PPE
by KEVIN KUO DDS, MMSc | May 7, 2020
The Federal Drug Administration (FDA) today banned most prior approved KN95 masks. The Emergency Use Authorization, which was initially placed on April 3rd, 2020, due to respirator shortages in the United States, was supposed to clear the way for respirators not approved by the National Institute of Occupational Safety and Health (NIOSH) to be used by healthcare personnel in the U.S. The FDA cited quality concerns for revising the EUA.
On May 7, 2020, in response to questions and concerns that have been received by FDA since issuance of the April 3, 2020 letter and having concluded that revising the April 3, 2020 EUA is appropriate to protect the public health or safety under section 564(g)(2)(C) of the Act (21 U.S.C. § 360bbb-3(g)(2)(C)), FDA is reissuing the April 3, 2020 letter with certain revisions.3 Specifically, FDA has revised the April 3, 2020 EUA for clarity and to address concerns about sub-standard products, which includes revising the third criterion for eligibility and adding a process for removal from Appendix A.
Tests have found many KN95 masks filtering less than 95% of 0.3-micron test particles. One Chinese manufacturer filtered out only 24% to 35%. The original list of non-NIOSH approved respirators (KN95s) included 80 Chinese manufacturers. Only 14 of the 80 are on the list today:
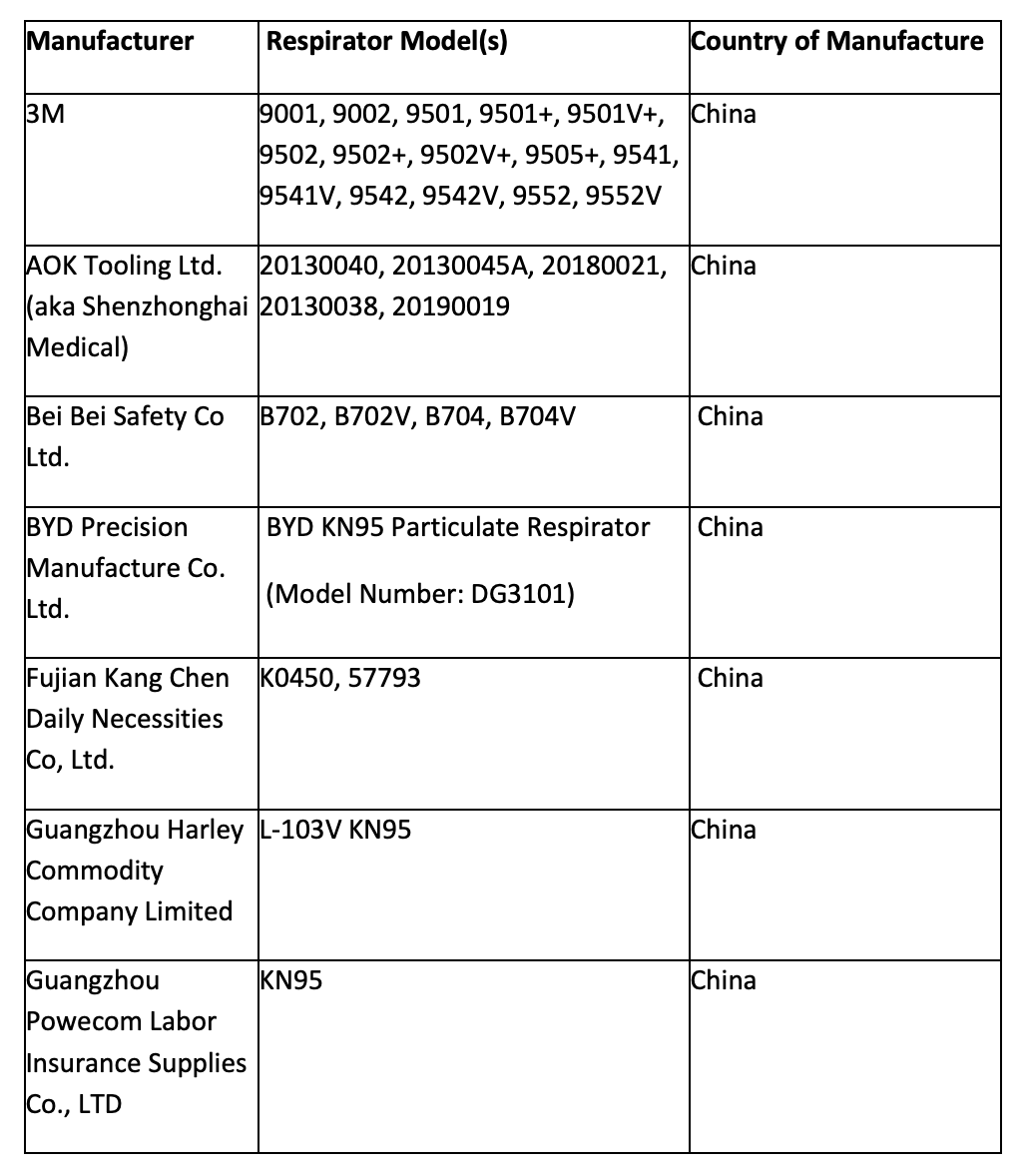
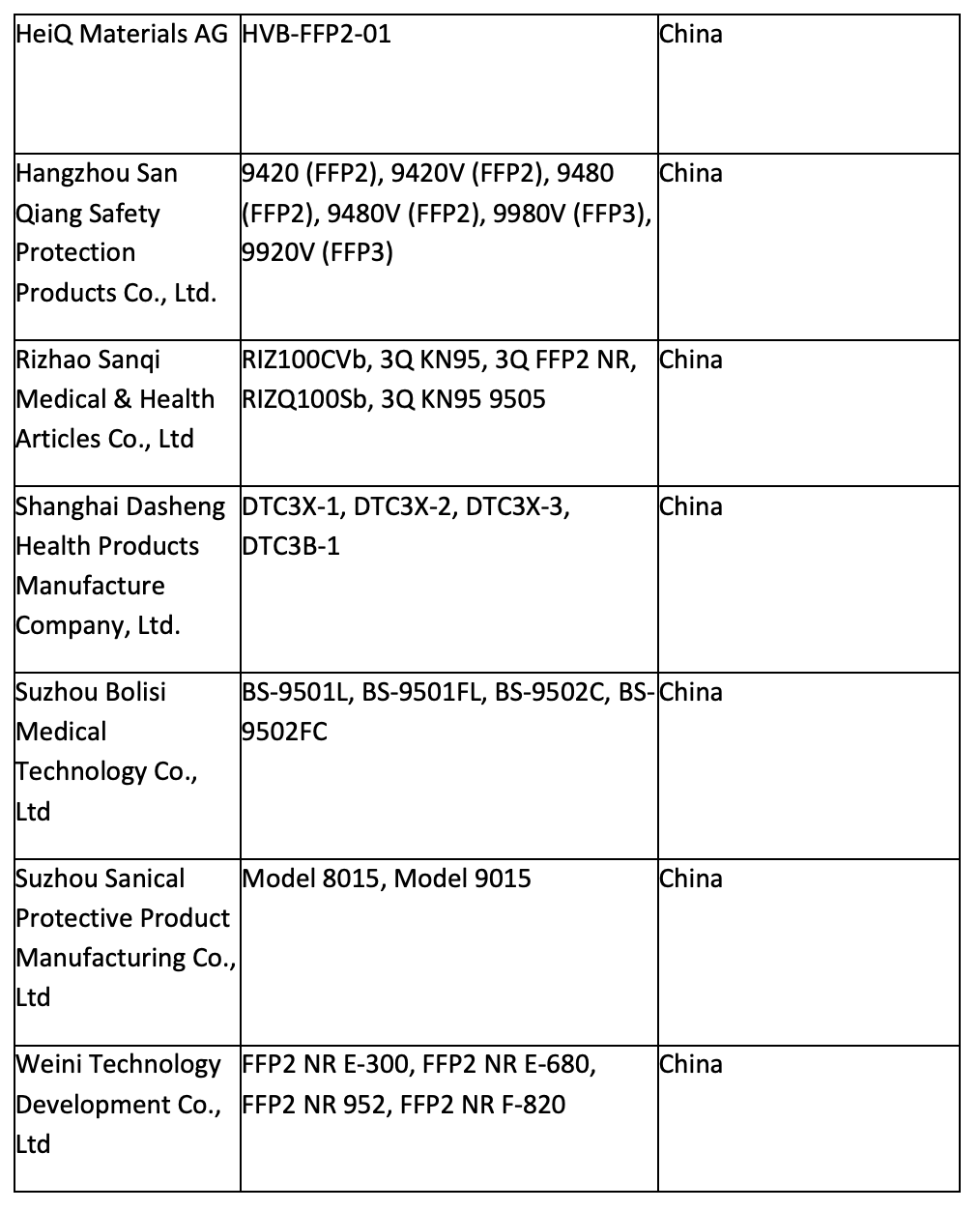
This announcement is bad news for dentists in the United States who purchased KN95 masks in place of unobtainable NIOSH-approved N95 masks. With an already overwhelming health safety concerns and financial constraints, this reversal of prior FDA-approved Chinese masks can set dental practices back even more with ownership of supplies now deemed unsafe by a federal regulatory body.





